Legal Representation
Attorney
Allison Strickland Ricketts
Application History
4 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Jan 27, 2026 | MAFR | O | APPLICATION FILING RECEIPT MAILED | Loading... |
| Jan 27, 2026 | MAFR | O | APPLICATION FILING RECEIPT MAILED | Loading... |
| Jan 27, 2026 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
| Jan 27, 2026 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
Detailed Classifications
Class 042
Pharmaceutical research and development services; conducting clinical trials for others for pharmaceutical preparations; pharmaceutical research services and pharmaceutical product evaluation, namely, conducting post-authorization research for the marketing of pharmaceutical preparations and evaluating epidemiological results; pharmaceutical research and development services in the field of neurological, central nervous system, and movement diseases and disorders and tardive dyskinesia; conducting clinical trials for others for pharmaceutical preparations for the treatment and prevention of neurological, central nervous system, and movement diseases and disorders and tardive dyskinesia; providing medical and scientific research information to patients and practitioners in the field of clinical trials, namely, as to the results of clinical trials; providing medical and scientific research information to patients and practitioners in the field of clinical trials, namely, as to the results of clinical trials pertaining to therapies and treatments for neurological, central nervous system, and movement diseases and disorders and tardive dyskinesia; pharmaceutical research services and pharmaceutical product evaluation, namely, conducting post-authorization research for the marketing of pharmaceutical preparations for the treatment and prevention of neurological, central nervous system, and movement diseases and disorders and tardive dyskinesia, and evaluating epidemiological results for treatments for neurological, central nervous system, and movement diseases and disorders and tardive dyskinesia
Class 044
Providing medical information; providing medical information relating to therapies and treatments for neurological, central nervous system, and movement diseases and disorders and tardive dyskinesia
Additional Information
Design Mark
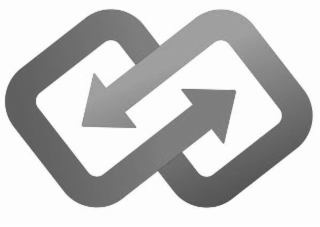
The mark consists of two interconnecting open rhombuses, the open edge of each rhombus ending in an arrowhead.
Design Mark
The mark consists of two interconnecting open rhombuses, the open edge of each rhombus ending in an arrowhead.
Color Claim
Color is not claimed as a feature of the mark.
Color Claim
Color is not claimed as a feature of the mark.
Classification
International Classes
042
044