Legal Representation
Attorney
Joseph E. Washington
USPTO Deadlines
Next Deadline
159 days remaining
NOA E-Mailed - SOU Required
Due Date
June 10, 2026
Extension Available
Until December 10, 2026
Application History
16 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Dec 10, 2025 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Dec 9, 2025 | EX1G | S | SOU EXTENSION 1 GRANTED | Loading... |
| Dec 9, 2025 | EXT1 | S | SOU EXTENSION 1 FILED | Loading... |
| Dec 9, 2025 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Jun 10, 2025 | NOAM | E | NOA E-MAILED - SOU REQUIRED FROM APPLICANT | Loading... |
| Apr 15, 2025 | NPUB | E | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED | Loading... |
| Apr 15, 2025 | PUBO | A | PUBLISHED FOR OPPOSITION | Loading... |
| Apr 9, 2025 | NONP | E | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED | Loading... |
| Mar 17, 2025 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Mar 17, 2025 | XAEC | I | EXAMINER'S AMENDMENT ENTERED | Loading... |
| Mar 17, 2025 | GNEN | O | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED | Loading... |
| Mar 17, 2025 | GNEA | F | EXAMINERS AMENDMENT E-MAILED | Loading... |
| Mar 17, 2025 | CNEA | R | EXAMINERS AMENDMENT -WRITTEN | Loading... |
| Mar 14, 2025 | DOCK | D | ASSIGNED TO EXAMINER | Loading... |
| Mar 13, 2025 | NWOS | I | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED | Loading... |
| Sep 3, 2024 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
Detailed Classifications
Class 005
Pharmaceutical preparations, namely, sodium thiosulfate used for the prevention of ototoxicity from cisplatin in pediatric patients
Additional Information
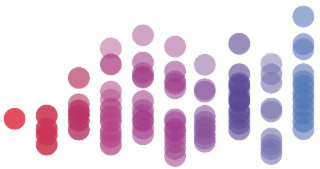
Design Mark
The mark consists of 10 columns comprised of varying amounts of circles per column. From left to right, the first through third columns are red, the fourth through sixth columns are magenta, the seventh column is purple, the eight column is dark purple, the ninth column is light purple, and the tenth column is blue.
Color Claim
The color(s) red, magenta, purple, dark purple, light purple, and blue is/are claimed as a feature of the mark.
Classification
International Classes
005