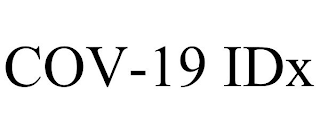Legal Representation
Attorney
Michael J. Tempel
USPTO Deadlines
Next Deadline
796 days remaining
Section 8 (6-Year) Declaration Due (Based on registration date 2021-09-28)
Due Date
September 28, 2027
Grace Period Ends
March 28, 2028
Additional deadlines exist. Contact your attorney for complete deadline information.
Application History
14 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Sep 28, 2021 | R.PR | A | REGISTERED-PRINCIPAL REGISTER | Loading... |
| Jul 13, 2021 | PUBO | A | PUBLISHED FOR OPPOSITION | Loading... |
| Jul 13, 2021 | NPUB | E | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED | Loading... |
| Jun 23, 2021 | NONP | E | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED | Loading... |
| Jun 5, 2021 | CNSA | O | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Jun 1, 2021 | TROA | I | TEAS RESPONSE TO OFFICE ACTION RECEIVED | Loading... |
| Jun 1, 2021 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
| Jun 1, 2021 | TEME | I | TEAS/EMAIL CORRESPONDENCE ENTERED | Loading... |
| Mar 14, 2021 | CNRT | R | NON-FINAL ACTION WRITTEN | Loading... |
| Mar 14, 2021 | GNRT | F | NON-FINAL ACTION E-MAILED | Loading... |
| Mar 14, 2021 | GNRN | O | NOTIFICATION OF NON-FINAL ACTION E-MAILED | Loading... |
| Feb 26, 2021 | DOCK | D | ASSIGNED TO EXAMINER | Loading... |
| Oct 19, 2020 | NWOS | I | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED | Loading... |
| Sep 15, 2020 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
Detailed Classifications
Class 005
Diagnostic kits comprised of medical diagnostic reagents and assays for testing of bodily fluids for use in disease detection, namely, SARS-CoV-2 (COVID-19); Diagnostic preparations for medical purposes; all the foregoing related to the diagnosis of COVID-19
First Use Anywhere:
Mar 15, 2020
First Use in Commerce:
Mar 15, 2020
Class 044
Medical analysis services for diagnostic and treatment purposes provided by medical laboratories; Medical diagnostic testing, monitoring and reporting services; Medical testing for diagnostic or treatment purposes in the field of SARS-CoV-2 (COVID-19); all the foregoing related to the treatment or diagnosis of COVID-19
First Use Anywhere:
Mar 15, 2020
First Use in Commerce:
Mar 15, 2020
Additional Information
Pseudo Mark
COV-NINETEEN IDX; COV-ONE NINE IDX
Classification
International Classes
005
044
Disclaimers
The following terms have been disclaimed and are not claimed as part of the trademark:
General Disclaimer
"COV-19"