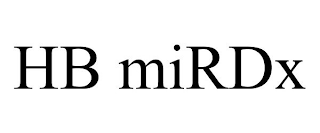Legal Representation
Attorney
Daniel A. Thomson
USPTO Deadlines
Application History
21 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Nov 15, 2021 | MAB6 | E | ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED | Loading... |
| Nov 15, 2021 | ABN6 | S | ABANDONMENT - NO USE STATEMENT FILED | Loading... |
| Jan 30, 2021 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Jan 28, 2021 | EX1G | S | SOU EXTENSION 1 GRANTED | Loading... |
| Jan 28, 2021 | EXT1 | S | SOU EXTENSION 1 FILED | Loading... |
| Jan 28, 2021 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Oct 13, 2020 | NOAM | E | NOA E-MAILED - SOU REQUIRED FROM APPLICANT | Loading... |
| Aug 18, 2020 | NPUB | E | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED | Loading... |
| Aug 18, 2020 | PUBO | A | PUBLISHED FOR OPPOSITION | Loading... |
| Jul 29, 2020 | NONP | E | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED | Loading... |
| Jul 15, 2020 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Jul 10, 2020 | TEME | I | TEAS/EMAIL CORRESPONDENCE ENTERED | Loading... |
| Jul 10, 2020 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
| Jul 8, 2020 | ALIE | A | ASSIGNED TO LIE | Loading... |
| Jun 26, 2020 | TROA | I | TEAS RESPONSE TO OFFICE ACTION RECEIVED | Loading... |
| Jun 10, 2020 | GNRN | O | NOTIFICATION OF NON-FINAL ACTION E-MAILED | Loading... |
| Jun 10, 2020 | GNRT | F | NON-FINAL ACTION E-MAILED | Loading... |
| Jun 10, 2020 | CNRT | R | NON-FINAL ACTION WRITTEN | Loading... |
| Jun 9, 2020 | DOCK | D | ASSIGNED TO EXAMINER | Loading... |
| Mar 21, 2020 | NWOS | I | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED | Loading... |
| Mar 20, 2020 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
Detailed Classifications
Class 010
medical diagnostic kits consisting primarily of medical diagnostic reagents for detection or quantitative analysis of BK virus infection or reactivation; medical diagnostic kits consisting primarily of medical diagnostic reagents for detection or quantitative analysis of kidney transplant rejection; medical diagnostic kits for consisting primarily of medical diagnostic reagents detection or quantitative analysis of Epstein-Barr virus infection; medical diagnostic kits consisting primarily of medical diagnostic reagents for detection or quantitative analysis of Cytomegalo virus infection; medical diagnostic kits for consisting primarily of medical diagnostic reagents prediction of targeted therapy efficacy for metastatic colorectal cancer; medical diagnostic kits consisting primarily of medical diagnostic reagents for prediction of targeted therapy efficacy for non-small cell lung cancer; medical diagnostic kits consisting primarily of medical diagnostic reagents for prognosis of liver cancer after liver transplant; medical diagnostic kits consisting primarily of medical diagnostic reagents for detection or quantitative analysis of liver cancer; medical diagnostic kits consisting primarily of medical diagnostic reagents for detection or quantitative analysis of pancreatic cancer; medical diagnostic kits consisting primarily of medical diagnostic reagents for detection or quantitative analysis of lung cancer
Additional Information
Pseudo Mark
HEIM BIO TECH MIRDX
Classification
International Classes
010