Legal Representation
Attorney
Stephanie K. Wade
USPTO Deadlines
Application History
50 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Apr 5, 2021 | MAB6 | E | ABANDONMENT NOTICE E-MAILED - NO USE STATEMENT FILED | Loading... |
| Apr 5, 2021 | ABN6 | S | ABANDONMENT - NO USE STATEMENT FILED | Loading... |
| Aug 1, 2020 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Jul 31, 2020 | EX5G | S | SOU EXTENSION 5 GRANTED | Loading... |
| Jul 29, 2020 | EXT5 | S | SOU EXTENSION 5 FILED | Loading... |
| Jul 29, 2020 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Feb 7, 2020 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Feb 5, 2020 | EX4G | S | SOU EXTENSION 4 GRANTED | Loading... |
| Feb 5, 2020 | EXT4 | S | SOU EXTENSION 4 FILED | Loading... |
| Feb 5, 2020 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Jan 30, 2020 | ARAA | I | ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED | Loading... |
| Jan 30, 2020 | REAP | I | TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED | Loading... |
| Jan 21, 2020 | ARAA | I | ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED | Loading... |
| Jan 21, 2020 | REAP | I | TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED | Loading... |
| Oct 16, 2019 | ARAA | I | ATTORNEY/DOM.REP.REVOKED AND/OR APPOINTED | Loading... |
| Oct 16, 2019 | REAP | I | TEAS REVOKE/APP/CHANGE ADDR OF ATTY/DOM REP RECEIVED | Loading... |
| Aug 22, 2019 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Aug 19, 2019 | EX3G | S | SOU EXTENSION 3 GRANTED | Loading... |
| Aug 19, 2019 | EXT3 | S | SOU EXTENSION 3 FILED | Loading... |
| Aug 19, 2019 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Feb 19, 2019 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Feb 15, 2019 | EX2G | S | SOU EXTENSION 2 GRANTED | Loading... |
| Feb 15, 2019 | EXT2 | S | SOU EXTENSION 2 FILED | Loading... |
| Feb 15, 2019 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Sep 18, 2018 | NOAC | E | CORRECTED NOA E-MAILED | Loading... |
| Sep 18, 2018 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Sep 17, 2018 | EX1G | S | SOU EXTENSION 1 GRANTED | Loading... |
| Aug 20, 2018 | EXT1 | S | SOU EXTENSION 1 FILED | Loading... |
| Sep 17, 2018 | DPCC | D | DIVISIONAL PROCESSING COMPLETE | Loading... |
| Aug 21, 2018 | DRRR | I | DIVISIONAL REQUEST RECEIVED | Loading... |
| Sep 17, 2018 | AITU | A | CASE ASSIGNED TO INTENT TO USE PARALEGAL | Loading... |
| Aug 21, 2018 | ERTD | I | TEAS REQUEST TO DIVIDE RECEIVED | Loading... |
| Aug 20, 2018 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Feb 27, 2018 | NOAM | E | NOA E-MAILED - SOU REQUIRED FROM APPLICANT | Loading... |
| Feb 20, 2018 | CHPB | I | POST PUBLICATION AMENDMENT - ENTERED | Loading... |
| Feb 16, 2018 | APET | A | ASSIGNED TO PETITION STAFF | Loading... |
| Feb 6, 2018 | EPPA | I | TEAS POST PUBLICATION AMENDMENT RECEIVED | Loading... |
| Jan 2, 2018 | NPUB | E | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED | Loading... |
| Jan 2, 2018 | PUBO | A | PUBLISHED FOR OPPOSITION | Loading... |
| Dec 13, 2017 | NONP | E | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED | Loading... |
| Nov 26, 2017 | ALIE | A | ASSIGNED TO LIE | Loading... |
| Nov 14, 2017 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Nov 14, 2017 | XAEC | I | EXAMINER'S AMENDMENT ENTERED | Loading... |
| Nov 14, 2017 | GNEN | O | NOTIFICATION OF EXAMINERS AMENDMENT E-MAILED | Loading... |
| Nov 14, 2017 | GNEA | O | EXAMINERS AMENDMENT E-MAILED | Loading... |
| Nov 14, 2017 | CNEA | R | EXAMINERS AMENDMENT -WRITTEN | Loading... |
| Nov 10, 2017 | ZZZX | Z | PREVIOUS ALLOWANCE COUNT WITHDRAWN | Loading... |
| Nov 10, 2017 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Nov 9, 2017 | TEME | I | TEAS/EMAIL CORRESPONDENCE ENTERED | Loading... |
| Nov 8, 2017 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
Detailed Classifications
Class 001
Biochemicals for scientific, laboratory, and research use, namely, proteolytically-activatable, protease-activatable, or proteolytically activated or protease-activated immunoglobulin and antigen-binding fragments for binding to tissue, cells and antigens; diagnostic kits for scientific, laboratory, and research use comprising proteolytically-activatable or protease-activatable, proteolytically-activated or protease-activated immunoglobulin and antigen-binding fragments for binding to tissue, cells, and antigens; immunoglobulin and antigen-binding fragments for drug conjugation and targeted cell binding for laboratory and research use; assays and reagents for use in scientific, laboratory and research purposes; biochemical test kits comprising assays and reagents for scientific, laboratory and research purposes; assay kits comprising assays and reagents for measuring, monitoring, testing and analyzing proteolytic activation and antibody binding for scientific, laboratory and research use
Class 005
Infections, inflammation; infections, inflammation; infections, inflammation; engineered cell therapies for medical purposes, namely, for use in treating cancer, infections, autoimmunity, and inflammation; infections, inflammation; assays and reagents for use in medical, medical diagnostic and therapeutic purposes for proteolytic activation and binding to tumor cells; biochemical test kits comprised of assays and reagents for medical, medical diagnostic and therapeutic purposes for proteolytic activation and binding to tumor cells; assay kits comprised of assays and reagents for measuring, monitoring, testing and analyzing proteolytic activation, antibody binding, therapeutic treatments and patient recovery for medical, medical diagnostic and therapeutic purposes; assay kits comprised of assays and reagents for use in identifying candidate patient populations for specific therapeutic treatments for medical purposes; therapeutic kits comprising proteolytically-activatable or protease-activatable, proteolytically-activated or protease-activated immunoglobulin and antigen-binding fragments for binding to tissue, cells, and antigens
Class 042
Scientific research and development in the field of therapeutic biochemicals, engineered cell therapies and immunotherapy, oncology and dysregulated proteolytic activity; medical and scientific research and development for agents, preparations, products and technology in the field of dysregulated proteolytic activities; providing medical and scientific research information in the fields of biochemicals, engineered cell therapies, immunotherapy, oncology and dysregulated proteolytic activity; scientific investigations for medical purposes; medical research; providing scientific and medical research information and analysis relating to biochemicals, engineered cell therapies and immunotherapy, proteolytic activation, antibody binding, therapeutic treatments, patient recovery, and the identification of candidate patients for specific therapeutic treatments; testing, research, design and development services in the field of biochemicals for scientific and medical research purposes; research, design and development of assays for measuring, monitoring, testing and analyzing proteolytic activation, antibody binding, therapeutic treatments and patient recovery, and for identifying candidate patient populations for specific therapeutic treatments; providing technical support and consultation services relating to antibody selection and use, namely, providing technical scientific research consultation services; online computer services in the nature of providing a search engine for obtaining data, namely, searching, retrieving, and providing information on antibodies and research topics pertaining to antibodies on a global computer network
Additional Information
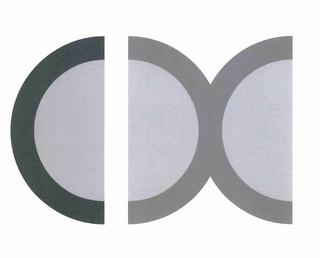
Design Mark
The mark consists of three semi-circles aligned next to each other. The first semi-circle has its vertical opening facing to the right. It is separated by a narrow white space from the second semi-circle, whose vertical opening faces to the left. The third semi-circle's vertical opening faces to the right. The tips of the circular apexes of the second and third semi circles are adjoined to each other.
Color Claim
Color is not claimed as a feature of the mark.
Classification
International Classes
001
005
042