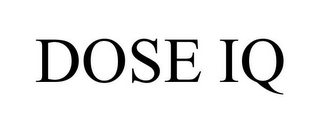Legal Representation
Attorney
JEFFREY C NICHOLS
USPTO Deadlines
Application History
9 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Jul 27, 2016 | MAB1 | O | ABANDONMENT NOTICE MAILED - EXPRESS ABANDONMENT | Loading... |
| Jul 27, 2016 | ABN1 | O | ABANDONMENT - EXPRESS MAILED | Loading... |
| Jul 26, 2016 | EXAR | I | TEAS EXPRESS ABANDONMENT RECEIVED | Loading... |
| Jun 24, 2016 | GNRN | O | NOTIFICATION OF NON-FINAL ACTION E-MAILED | Loading... |
| Jun 24, 2016 | GNRT | F | NON-FINAL ACTION E-MAILED | Loading... |
| Jun 24, 2016 | CNRT | R | NON-FINAL ACTION WRITTEN | Loading... |
| Jun 23, 2016 | DOCK | D | ASSIGNED TO EXAMINER | Loading... |
| Jun 14, 2016 | NWOS | I | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED | Loading... |
| Jun 10, 2016 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
Detailed Classifications
Class 009
Database software for use with parenteral infusion devices for monitoring administration of pharmaceutical compositions; database software for use with parenteral infusion devices for defining medication delivery parameters of pharmaceutical compositions; database software for use with parenteral infusion devices for monitoring the programming of the infusion device by a clinician; computer software for use with parenteral infusion devices for monitoring and controlling dose limits and indicating suggested concentrations, volumes and drug amounts based on the hospital protocol
First Use Anywhere:
0
First Use in Commerce:
0
Classification
International Classes
009