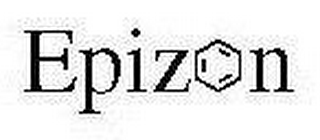Legal Representation
Attorney
Alexander P. Montgomery
USPTO Deadlines
Next Deadline
1139 days remaining
Section 8 & 9 Renewal Due (Principal Register) (Based on registration date 20190305)
Due Date
March 05, 2029
Grace Period Ends
September 05, 2029
Application History
49 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Jan 31, 2025 | NA85 | E | NOTICE OF ACCEPTANCE OF SEC. 8 & 15 - E-MAILED | Loading... |
| Jan 31, 2025 | C15A | O | REGISTERED - SEC. 8 (6-YR) ACCEPTED & SEC. 15 ACK. | Loading... |
| Jan 31, 2025 | APRE | A | CASE ASSIGNED TO POST REGISTRATION PARALEGAL | Loading... |
| Sep 13, 2024 | E815 | I | TEAS SECTION 8 & 15 RECEIVED | Loading... |
| Mar 5, 2024 | REM1 | E | COURTESY REMINDER - SEC. 8 (6-YR) E-MAILED | Loading... |
| Mar 5, 2019 | R.PR | A | REGISTERED-PRINCIPAL REGISTER | Loading... |
| Jan 31, 2019 | SUNA | E | NOTICE OF ACCEPTANCE OF STATEMENT OF USE E-MAILED | Loading... |
| Jan 30, 2019 | CNPR | P | ALLOWED PRINCIPAL REGISTER - SOU ACCEPTED | Loading... |
| Jan 4, 2019 | MDSC | E | NOTICE OF DESIGN SEARCH CODE E-MAILED | Loading... |
| Jan 3, 2019 | SUPC | I | STATEMENT OF USE PROCESSING COMPLETE | Loading... |
| Dec 19, 2018 | IUAF | S | USE AMENDMENT FILED | Loading... |
| Jan 3, 2019 | DPCC | D | DIVISIONAL PROCESSING COMPLETE | Loading... |
| Dec 19, 2018 | DRRR | I | DIVISIONAL REQUEST RECEIVED | Loading... |
| Dec 19, 2018 | ERTD | I | TEAS REQUEST TO DIVIDE RECEIVED | Loading... |
| Dec 19, 2018 | EISU | I | TEAS STATEMENT OF USE RECEIVED | Loading... |
| Nov 29, 2018 | EWAF | I | TEAS WITHDRAWAL OF ATTORNEY RECEIVED-FIRM RETAINS | Loading... |
| May 29, 2018 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| May 25, 2018 | EX3G | S | SOU EXTENSION 3 GRANTED | Loading... |
| May 25, 2018 | EXT3 | S | SOU EXTENSION 3 FILED | Loading... |
| May 25, 2018 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Nov 29, 2017 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| Nov 27, 2017 | EX2G | S | SOU EXTENSION 2 GRANTED | Loading... |
| Nov 27, 2017 | EXT2 | S | SOU EXTENSION 2 FILED | Loading... |
| Nov 27, 2017 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| Jun 2, 2017 | EXRA | E | NOTICE OF APPROVAL OF EXTENSION REQUEST E-MAILED | Loading... |
| May 31, 2017 | EX1G | S | SOU EXTENSION 1 GRANTED | Loading... |
| May 31, 2017 | EXT1 | S | SOU EXTENSION 1 FILED | Loading... |
| May 31, 2017 | EEXT | I | SOU TEAS EXTENSION RECEIVED | Loading... |
| May 31, 2017 | TCCA | I | TEAS CHANGE OF CORRESPONDENCE RECEIVED | Loading... |
| May 5, 2017 | CHAN | I | APPLICANT/CORRESPONDENCE CHANGES (NON-RESPONSIVE) ENTERED | Loading... |
| May 1, 2017 | AITU | A | CASE ASSIGNED TO INTENT TO USE PARALEGAL | Loading... |
| Apr 11, 2017 | EWAF | I | TEAS WITHDRAWAL OF ATTORNEY RECEIVED-FIRM RETAINS | Loading... |
| Dec 27, 2016 | NOAM | E | NOA E-MAILED - SOU REQUIRED FROM APPLICANT | Loading... |
| Nov 1, 2016 | NPUB | E | OFFICIAL GAZETTE PUBLICATION CONFIRMATION E-MAILED | Loading... |
| Nov 1, 2016 | PUBO | A | PUBLISHED FOR OPPOSITION | Loading... |
| Oct 12, 2016 | NONP | E | NOTIFICATION OF NOTICE OF PUBLICATION E-MAILED | Loading... |
| Sep 26, 2016 | PREV | O | LAW OFFICE PUBLICATION REVIEW COMPLETED | Loading... |
| Sep 20, 2016 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Sep 1, 2016 | TEME | I | TEAS/EMAIL CORRESPONDENCE ENTERED | Loading... |
| Sep 1, 2016 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
| Aug 25, 2016 | ALIE | A | ASSIGNED TO LIE | Loading... |
| Aug 19, 2016 | TROA | I | TEAS RESPONSE TO OFFICE ACTION RECEIVED | Loading... |
| Mar 11, 2016 | GNRN | O | NOTIFICATION OF NON-FINAL ACTION E-MAILED | Loading... |
| Mar 11, 2016 | GNRT | F | NON-FINAL ACTION E-MAILED | Loading... |
| Mar 11, 2016 | CNRT | R | NON-FINAL ACTION WRITTEN | Loading... |
| Mar 9, 2016 | DOCK | D | ASSIGNED TO EXAMINER | Loading... |
| Nov 21, 2015 | MDSC | E | NOTICE OF DESIGN SEARCH CODE E-MAILED | Loading... |
| Nov 20, 2015 | NWOS | I | NEW APPLICATION OFFICE SUPPLIED DATA ENTERED | Loading... |
| Nov 20, 2015 | NWAP | I | NEW APPLICATION ENTERED | Loading... |
Detailed Classifications
Class 005
pharmaceutical preparations for the treatment of vascular disease, cardiovascular disease, heart disease, osteoporosis, bone disease, renal disease, kidney disease, autoimmune disease, atherosclerosis, excluding devices or drug systems for the delivery of pharmaceuticals for the treatment of allergic reactions, anaphylaxis, or asthma and pharmaceuticals for the treatment of allergic reaction, anaphylaxis or asthma
First Use Anywhere:
Jul 30, 2018
First Use in Commerce:
Jul 30, 2018
Additional Information
Design Mark
The mark consists of the word "EPIZON" featuring a benzene ring as the "O".
Color Claim
Color is not claimed as a feature of the mark.
Classification
International Classes
005