Legal Representation
Attorney
M ROBERT KESTENBAUM
USPTO Deadlines
Application History
18 events| Date | Code | Type | Description | Documents |
|---|---|---|---|---|
| Nov 5, 2003 | ABN6 | S | ABANDONMENT - NO USE STATEMENT FILED | Loading... |
| Mar 18, 2003 | NOAM | O | NOA MAILED - SOU REQUIRED FROM APPLICANT | Loading... |
| Dec 24, 2002 | PUBO | A | PUBLISHED FOR OPPOSITION | Loading... |
| Dec 4, 2002 | NPUB | O | NOTICE OF PUBLICATION | Loading... |
| Oct 11, 2002 | 1.BA | I | Sec. 1(B) CLAIM ADDED | Loading... |
| May 10, 2002 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Mar 24, 2002 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
| Oct 19, 2001 | CNRT | O | NON-FINAL ACTION MAILED | Loading... |
| Oct 17, 2001 | ZZZX | Z | PREVIOUS ALLOWANCE COUNT WITHDRAWN | Loading... |
| May 3, 2001 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Dec 26, 2000 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
| Dec 26, 2000 | 44DD | I | SEC. 44(D) CLAIM DELETED | Loading... |
| Feb 2, 2001 | CNSI | O | INQUIRY AS TO SUSPENSION MAILED | Loading... |
| Feb 2, 2001 | ZZZX | Z | PREVIOUS ALLOWANCE COUNT WITHDRAWN | Loading... |
| Jan 8, 1999 | CNSA | P | APPROVED FOR PUB - PRINCIPAL REGISTER | Loading... |
| Nov 24, 1998 | CRFA | I | CORRESPONDENCE RECEIVED IN LAW OFFICE | Loading... |
| May 26, 1998 | CNRT | F | NON-FINAL ACTION MAILED | Loading... |
| May 19, 1998 | DOCK | D | ASSIGNED TO EXAMINER | Loading... |
Detailed Classifications
Class 001
diagnostic reagents for scientific or research use
First Use Anywhere:
0
First Use in Commerce:
0
Class 005
therapeutic pharmaceutical preparations for the treatment of skeletal diseases, connective tissue diseases, neurological diseases, urological diseases, kardiovascular diseases, dermatological diseases, liver diseases, autoimmune diseases; diagnostic reagents for medical or clinical laboratory use; live bones of any type and origin and live bone cells for subsequent implantation and transplantation; live tissue transplants and implants, namely, stemm cells, connective tissue, reproductive tissue, bone cells, skin cells, neuronal cells, smooth muscle cells, myofibroblasts, liver cells, epithelial cells, endothelial cells; support materials for bone cell transplants as pharmaceutical preparations, namely, organic and inorganic materials of synthetic and natural origin, namely, synthetic organic materials as poly (alpha-hydroxy-acids), polyethylene glycol, polyesters, polyuerethans, gelatine, alginates, resins, synthetic inorganic materials as hydroxyapatit, tricalcium phosphate, plaster of Paris, titanium, silicon, siloxanes, silanes, glass, ceramics, organic materials of natural origin as collagen, fibrin, bone extracts, cellulose, inorganic materials of natural origin as coralline hydroxyapatite, bovine bone matrix
First Use Anywhere:
0
First Use in Commerce:
0
Class 009
multimedia software recorded on CD-ROM containing advertising, informational and teaching materials for use in medical transplant procedures
First Use Anywhere:
0
First Use in Commerce:
0
Class 010
medical and surgical appliances and instruments, namely, scalpels, syringes, arthroskopical instruments, endoscopical instruments, forceps, suction punches, scissors; medical containers for transporting bone cells for use in medical transplant procedures
First Use Anywhere:
0
First Use in Commerce:
0
Class 016
printed materials, namely, books and instructional manuals containing advertising, information and teaching materials for use in medical transplant procedures
First Use Anywhere:
0
First Use in Commerce:
0
Class 039
transportation of autologous materials of others
First Use Anywhere:
0
First Use in Commerce:
0
Class 041
educational services, namely, seminars, courses and workshop featuring transplanting bone cells and organ transplants; teaching in the field of transplanting bone cells and organ transplants
First Use Anywhere:
0
First Use in Commerce:
0
Class 042
medical research and clinical studies of bone cells; medical services, namely, biological, biochemical, cell-biological, and molecular-biological preparation of bone cells for subsequent transplantation and implantation of bone cells in humans and animals, transplantation and implantation of cells in humans and animals, orthopedic services, medical trauma bone treatment services, plastic surgery; sports medicine services, and tissue engineering
First Use Anywhere:
0
First Use in Commerce:
0
Additional Information
Pseudo Mark
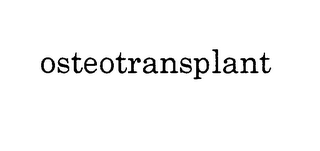
OSTEOTRANSPLANT
Classification
International Classes
001
005
009
010
016
039
041
042